www.aljazeerah.info
News, February 2020
Archives
Mission & Name
Conflict Terminology
Editorials
Gaza Holocaust
Gulf War
Isdood
Islam
News
News Photos
Opinion Editorials
US Foreign Policy (Dr. El-Najjar's Articles)
www.aljazeerah.info
|
Editorial Note: The following news reports are summaries from original sources. They may also include corrections of Arabic names and political terminology. Comments are in parentheses. |
2.4 Million Deaths and 112 Million Corona Virus Infections, Mostly in the US, Brazil, Mexico, India, UK, Italy, France, Russia, Germany, Spain
February 22, 2020
 |
 |
As of February 22, 2021, 16:31 GMT
World 112,088,404 infection cases, and 2,480,530 deaths.
A list of countries with the highest Coronavirus (Covid-19) deaths:
***
1 USA 28,769,583 infection cases, and 511,322 deaths.
2 Brazil 10,168,174 infection cases, and 246,560 deaths.
3 Mexico 2,041,380 infection cases, and 180,107 deaths.
4 India 11,008,665 infection cases, and 156,457 deaths.
5 UK 4,126,150 infection cases, and 120,757 deaths.
6
Italy 2,818,863 infection cases, and
95,992 deaths.
7
France 3,605,181 infection cases, and
84,306 deaths.
8 Russia 4,177,330 infection cases, and 83,630 deaths.
9 Germany 2,396,241 infection cases, and 68,479 deaths.
10 Spain 3,133,122 infection cases, and
67,101 deaths.
11
Iran 1,582,275 infection cases, and
59,572 deaths.
12 Colombia 2,226,262 infection cases, and 58,834 deaths.
13
Argentina 2,064,334 infection cases, and
51,198 deaths.
16
South Africa 1,503,796 infection cases, and
49,053 deaths.
17 Peru 1,283,309 infection cases, and 45,097 deaths.
18 Poland 1,642,658 infection cases, and 42,188 deaths.
***
WHO Director-General's opening remarks at press conference with H.E. Frank-Walter Steinmeier, Federal President of Germany - 22 February 2021
WHO, 22 February 2021
Vielen dank, Herr Bundespräsident, for your kind words, and thank you so much for your leadership and partnership.
As His Excellency said, we have just had a very constructive discussion about the global response to the COVID-19 pandemic, and about how we can strengthen the relationship between Germany and WHO even further.
Germany and WHO have had a strong relationship for many years, but that relationship has become even stronger in recent years.
I very much appreciate the leadership of Federal President Steinmeier, Chancellor Merkel and Minister Spahn during the pandemic, and their support for WHO and for global health.
Your support has given us as WHO inspiration and energy. Vielen dank, indeed, thank you so much.
Last year, Germany contributed more than US$600 million to WHO, including more than US$430 million to support the COVID-19 response.
Germany is also the largest donor to the WHO Contingency Fund for Emergencies, which enables WHO to respond rapidly to emergencies.
Germany was one of the main drivers behind the formation of the Access to COVID-19 Tools Accelerator last year, that was designed as an “endgame” to this pandemic, and has continued to be a leading advocate for solidarity and equity in the global response.
We very much appreciate Germany’s announcement of €1.5 billion in funding for the multilateral response to the pandemic, including the ACT Accelerator, at the G7 leaders’ meeting last week.
I also admire that Germany has been learning its own lessons from the pandemic – all of us have to learn from it, this pandemic is unprecedented – and I welcome the announcement by Chancellor Merkel last year that her government will invest 4 billion euros by 2026 in strengthening Germany’s public health capacities.
President Steinmeier and I also had a very good discussion about ways to strengthen WHO and global health security, including the International Treaty on Pandemic Preparedness and Response that has been proposed by Charles Michel, the President of the European Council. I would like to use this opportunity to thank my friend Charlie.
This proposed treaty would provide the framework to strengthen collaboration between nations and people.
We also discussed ongoing plans to establish a WHO centre for public health intelligence and risk analysis in Berlin, which I agreed with Chancellor Merkel last year.
So Your Excellency, vielen dank once again for your leadership and your partnership. We very much appreciate it, and we look forward to continuing to work with Germany very closely in the months and years ahead as we respond to the pandemic and work together for a healthier, safer, fairer world.
Thank you so much. Vielen dank.
WHO Director-General's opening remarks at press conference with H.E. Frank-Walter Steinmeier, Federal President of Germany - 22 February 2021
***
WHO Director-General's opening remarks at the media briefing on COVID-19 – 22 February 2021
WHO, 22 February 2021
I am deeply saddened and extremely concerned by the attack today on a humanitarian convoy in the Democratic Republic of the Congo which left three people dead, including the Italian Ambassador to that country. On Friday, leaders from several G7 countries and the European Union committed 4.3 billion U.S. dollars in new funding to finance the equitable distribution of vaccines, diagnostics and therapeutics for COVID-19. The G7 countries have shown leadership, but we need all countries to step up. We still face a gap of at least 22.9 billion dollars to fully finance the ACT Accelerator this year. Money is not the only challenge we face. If there are no vaccines to buy, money is irrelevant. Even if we have the funds, we can only deliver vaccines to poorer countries if high-income countries cooperate in respecting the deals COVAX has done, and the new deals it is doing.
---------------------------------------------------------------------------------------------------------------
Good morning, good afternoon and good evening.
I am deeply saddened and extremely concerned by the attack today on a humanitarian convoy in the Democratic Republic of the Congo which left three people dead, including the Italian Ambassador to that country.
I would like to express my deepest condolences to their families as well as to the government and people of Italy.
On Friday, leaders from several G7 countries and the European Union committed 4.3 billion U.S. dollars in new funding to finance the equitable distribution of vaccines, diagnostics and therapeutics for COVID-19.
Several G7 countries also committed to sharing doses with COVAX.
I would like to express my deep thanks to the G7 leaders for these contributions.
These funds and donations move us one step closer to meeting our target to start vaccination of health workers and older people in all countries within the first 100 days of the year.
The G7 countries have shown leadership, but we need all countries to step up.
We still face a gap of at least 22.9 billion dollars to fully finance the ACT Accelerator this year.
It’s important to note, however, that money is not the only challenge we face.
If there are no vaccines to buy, money is irrelevant.
Currently, some high-income countries are entering contracts with vaccine manufacturers that undermine the deals that COVAX has in place, and reduce the number of doses COVAX can buy.
Even if we have the funds, we can only deliver vaccines to poorer countries if high-income countries cooperate in respecting the deals COVAX has done, and the new deals it is doing.
This is not a matter of charity. It’s a matter of epidemiology.
Unless we end the pandemic everywhere, we will not end it anywhere.
The longer the virus circulates, the more opportunity it has to change in ways that could make vaccines less effective.
So it’s in the interest of all countries, including high-income countries, to ensure that health workers, older people and other at-risk groups are first in line for vaccines globally.
To achieve this, we need more funding, we need countries to share doses immediately, we need manufacturers to prioritize contracts with COVAX, and we also need a significant increase in the production of vaccines.
Recently I had a very productive discussion with President Emmanuel Macron of France, and I would like to thank him for his commitment to share 5% of France’s doses with COVAX.
More vaccines are being developed, approved and produced. There will be enough for everyone.
But for now and for the rest of this year, vaccines will be a limited resource. We must use them as strategically as we can.
Tomorrow I will be speaking at the Columbia University symposium on COVID-19 vaccine development, strategy and implementation.
Today I’m delighted to be joined by Lee Bollinger, the President of Columbia University.
The first major speech I gave after my election as Director-General in 2017 was at Columbia University, at the invitation of President Bollinger.
In that speech, I said that we do not know where or when the next global pandemic will occur, but we do know that it will exact a terrible toll, both on human life, and on the global economy.
More than three years later, we are unfortunately learning that lesson the hard way.
So President Bollinger, thank you so much for joining us today. You have the floor.
[PRESIDENT BOLLINGER ADDRESSES THE MEDIA]
Thank you, President Bollinger.
Our next guest needs no introduction. Dr Tony Fauci is one of the best-known names in global health, and for good reason.
For decades, Dr Fauci has not only been one of the world’s leading infectious disease experts, he has also been a fearless defender and articulate explainer of science and public health.
My friend Tony, thank you for your leadership over so many years, and especially during the past year. And thank you for joining us today. You have the floor.
[DR FAUCI ADDRESSES THE MEDIA]
Thank you so much, Tony.
And finally, it’s my honour to welcome Dr Nancy Messonnier, the Director of the National Center for Immunization and Respiratory Diseases at the U.S. Centers for Disease Control and Prevention.
Dr Messonnier is leading the CDC’s efforts on COVID-19 vaccination, and is one of the world’s leading experts on vaccination.
Among many other achievements, she played a vital role in the development and implementation of a low-cost vaccine to prevent epidemic meningococcal meningitis in Africa.
Dr Messonnier, thank you so much for joining us today. You have the floor.
[DR MESSONNIER ADDRESSES THE MEDIA]
Thank you, Dr Messonnier. And thank you to all of our guests for joining us today.
As we often say, it’s not vaccines that save lives, it’s vaccination.
In 1796, Edward Jenner administered the first vaccine against smallpox.
It took another 184 years for smallpox to be eradicated.
In combination with proven public health measures, vaccines give us the tools to control COVID-19. Whether we can is no longer a test of science; it’s a test of character.
Fadéla, back to you.
WHO Director-General's opening remarks at the media briefing on COVID-19 – 22 February 2021
***
Country readiness for COVID-19 vaccines
WHO, 19 February 2021
This article is part six in a series of explainers on vaccine development and distribution.
Part one focused on how vaccines work to protect our bodies from disease-carrying germs. Part two focused on the ingredients in a vaccine and the three clinical trial phases. Part three focused on the steps from completing the clinical trial phases through to distribution. Part four focused on the different types of vaccines. Part five focused on fair and equitable distribution of vaccines.
This document outlines the next part of the vaccine journey: how countries are getting ready for COVID-19 vaccines.
Countries are beginning to deploy COVID-19 vaccines, bringing new hope to the fight against the global pandemic. WHO, UNICEF, Gavi and many other partners are working together to support countries in preparing for COVID-19 vaccine introduction. They have provided a COVID-19 vaccine introduction toolbox that has all the resources a country needs to get ready for delivering COVID-19 vaccines. Within this toolbox, training is available for national/subnational focal points and health workers to equip them with the necessary knowledge and skills.
What is the process for countries to get COVID-19 vaccines from the COVAX Facility?
A total of 92 low- and middle-income economies will be able to access COVID-19 vaccines through the COVAX Facility Advance Market Commitment (AMC).
The COVAX AMC is the innovative financing instrument that will support the participation of 92 low- and middle-income economies in the COVAX Facility – enabling access to donor-funded doses of safe and effective COVID-19 vaccines. The AMC, combined with additional support for country readiness and delivery, will make sure the most vulnerable in all countries can be protected in the short term, regardless of income level.
These AMC92 countries must develop a COVID-19 National Deployment and Vaccination Plans (NDVPs) which is reviewed by WHO, UNICEF and other partners to help the country be sure the plan is as good as it can be. NDVPs can be submitted through the Partners Platform.
Once a country's NDVP is reviewed to ensure the key readiness criteria are met, they can be allocated vaccines through the COVAX Facility. Non-AMC countries are also welcome to submit their NDVP for review and receive feedback to strengthen their plan.
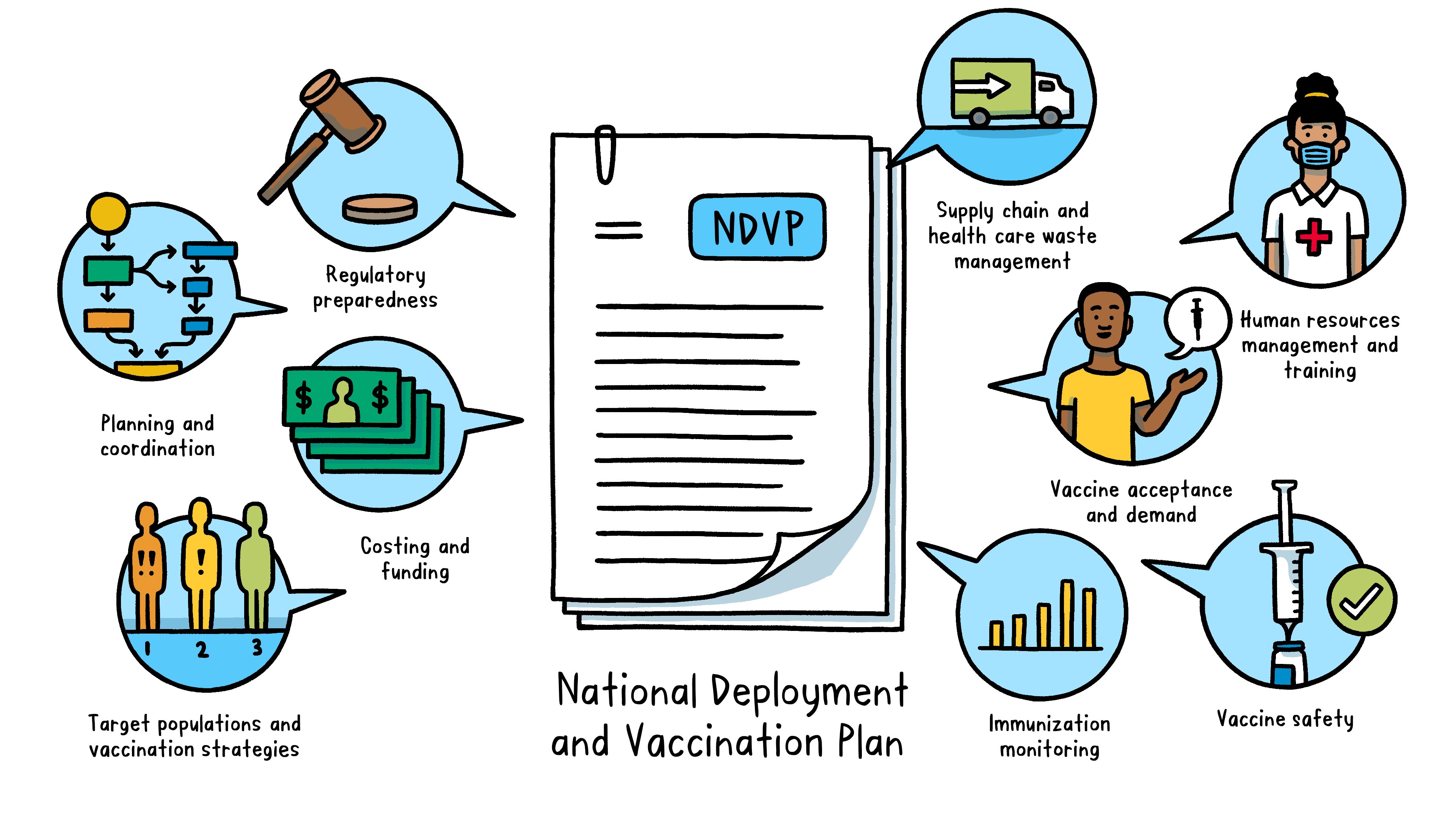
What is a National Deployment and Vaccination Plan?
A National Deployment and Vaccination Plan is an operational plan to implement and monitor COVID-19 vaccination rollout in a country. The NDVP serves as the “one-country plan” and main framework for a country’s vaccine introduction and vaccination efforts.
The NDVP outlines key aspects of readiness, including:
Regulatory preparedness: legal requirements for importing vaccines, regulatory authorization for using vaccines, and procedures to expedite vaccine availability Planning and coordination: governance and management structure at both country (such as a national coordinating committee and national immunization technical advisory groups) and subnational levels to oversee the deployment, implementation and monitoring of COVID-19 vaccines Costing and funding: a realistic budget – including funding sources and budget gaps – for vaccine deployment and vaccination Target populations and vaccination strategies: order of priority for different population groups to receive vaccination and how each group will be vaccinated Supply chain and health care waste management: critical supply chain activities to prepare for vaccine deployment (such as cold chain assessment and secure distribution and logistics) and to ensure safe handling of health care waste Human resources management and training: staff requirements for the vaccine rollout and a plan for training and supervision Vaccine acceptance and demand: an integrated approach to achieve high uptake of COVID-19 vaccines, including strategic communication activities that promote vaccination, manage misinformation and convey risk in the event of any adverse effects Vaccine safety: steps to prepare for, monitor and address vaccine safety and potential adverse events and ensure injection safety Immunization monitoring: a strategy for collection and reporting of immunization data, disease surveillance to monitor vaccine impact, and evaluation of the overall implementation process
Each country’s NDVP should be developed through a consultative process, led by the country’s Ministry of Health and supported by other organizations, including WHO, UNICEF and other relevant partners, to fine-tune the plan until it is complete. The Country Readiness and Delivery workstream of the ACT Accelerator has issued guidance on developing a national deployment and vaccination plan for COVID-19 vaccines, which helps countries produce their plan for COVID-19 vaccine introduction.
Why should countries develop a National Deployment and Vaccination Plan?
Developing an NDVP helps countries prepare for COVID-19 vaccination, identify resource needs and streamline the process for introducing vaccines. Once an NDVP is developed, countries work on its implementation, including the detailed planning of vaccine delivery strategies, logistics, HR and funding mobilization.
How will country readiness be assessed?
A review process has been set up to provide support and feedback to countries for improving and finalizing their plans. This ensures that a country’s NDVP includes the necessary elements to rapidly deploy vaccines and implement COVID-19 vaccination, and that any recommendations for improvement are addressed.
Once an AMC92 participant submits its NDVP, a Regional Review Committee (RRC) assesses the plan and evaluates country preparedness and capacity for vaccine allocation, deployment and administration. Those participants with an NDVP that meets the standardized readiness assessment join the process for allocating COVID-19 vaccines from the COVAX Facility. Countries should take into account the recommendations from the review to further improve their preparedness for vaccine introduction.
Country readiness for COVID-19 vaccines (who.int)
***
Share the link of this article with your facebook friendsFair Use Notice
This site contains copyrighted material the
use of which has not always been specifically authorized by the copyright
owner. We are making such material available in our efforts to advance
understanding of environmental, political, human rights, economic,
democracy, scientific, and social justice issues, etc. We believe this
constitutes a 'fair use' of any such copyrighted material as provided for
in section 107 of the US Copyright Law. In accordance with Title 17 U.S.C.
Section 107, the material on this site is
distributed without profit to those
who have expressed a prior interest in receiving the included information
for research and educational purposes. For more information go to: http://www.law.cornell.edu/uscode/17/107.shtml.
If you wish to use copyrighted material from this site for purposes of
your own that go beyond 'fair use', you must obtain permission from the
copyright owner.
|
|
|
|
||
|
||||||


